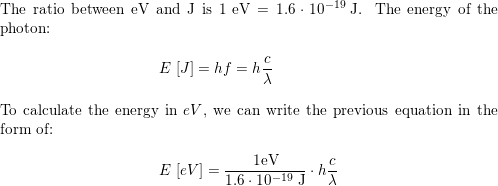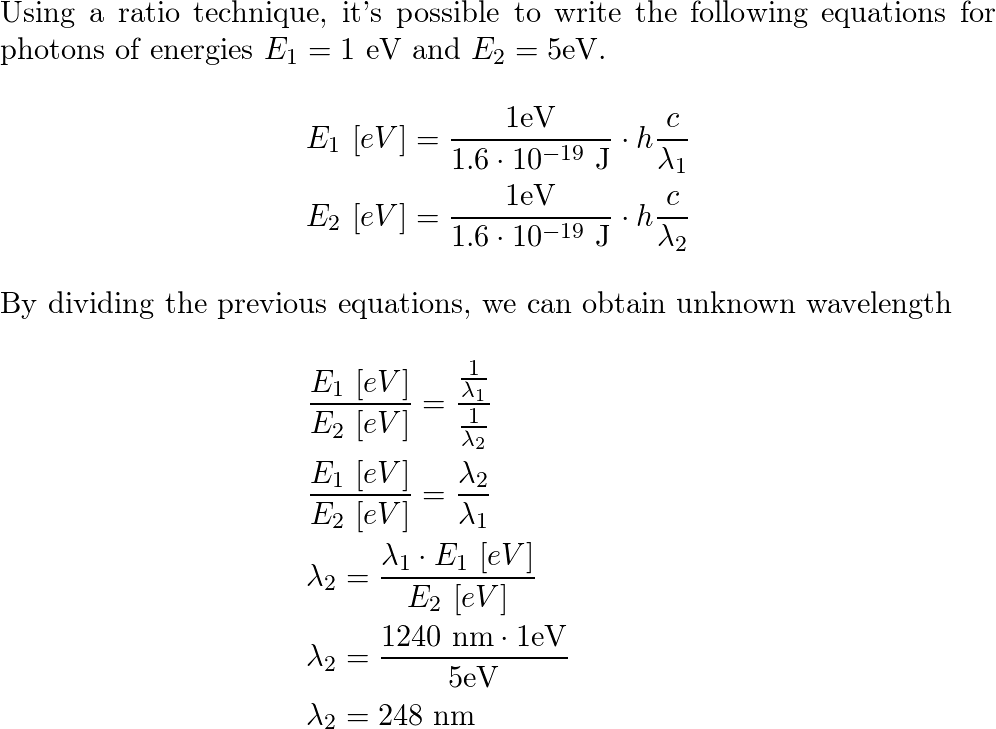Electric Vehicle Conversion Kits 145kw 155kwkw 300nm 310nm Electric Car Conversion Kit 2t 2.5t Complete Kit for Automotive Electric Motor - China Electric Vehicle Motor, AC Induction Motor | Made-in-China.com
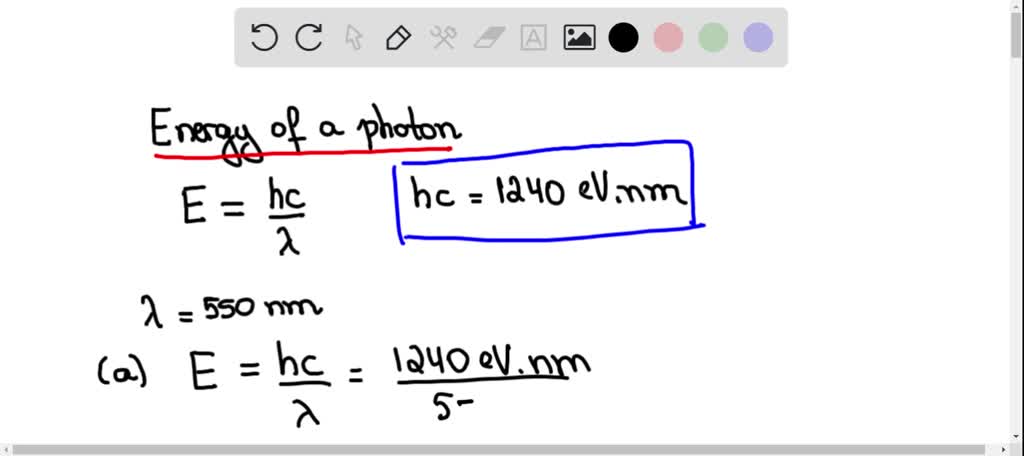
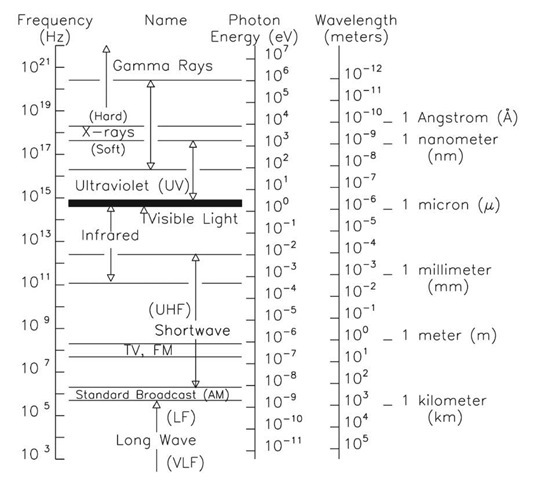
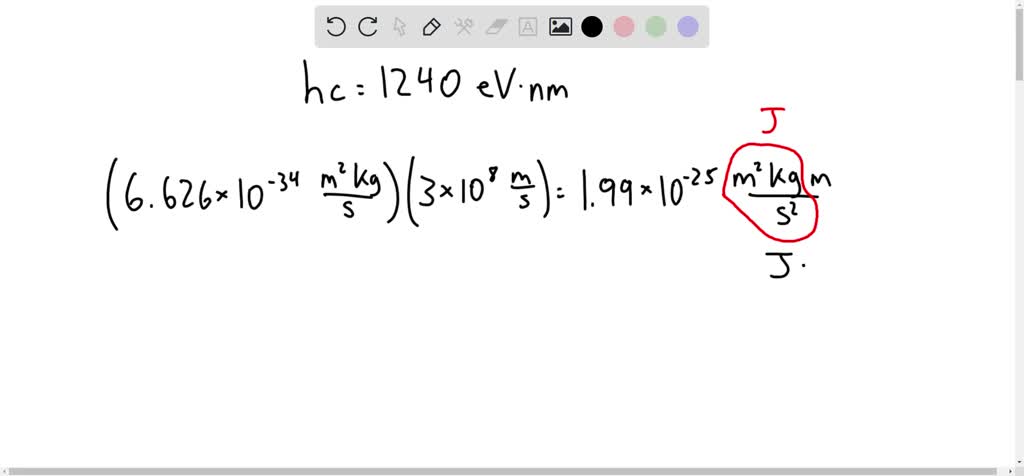
A firefly glows by the direct conversion of chemical energy to light. The light emitted by a firefly has peak intensity at a wavelength of 550 nm a. What is the minimum chemical energy, in eV, ...

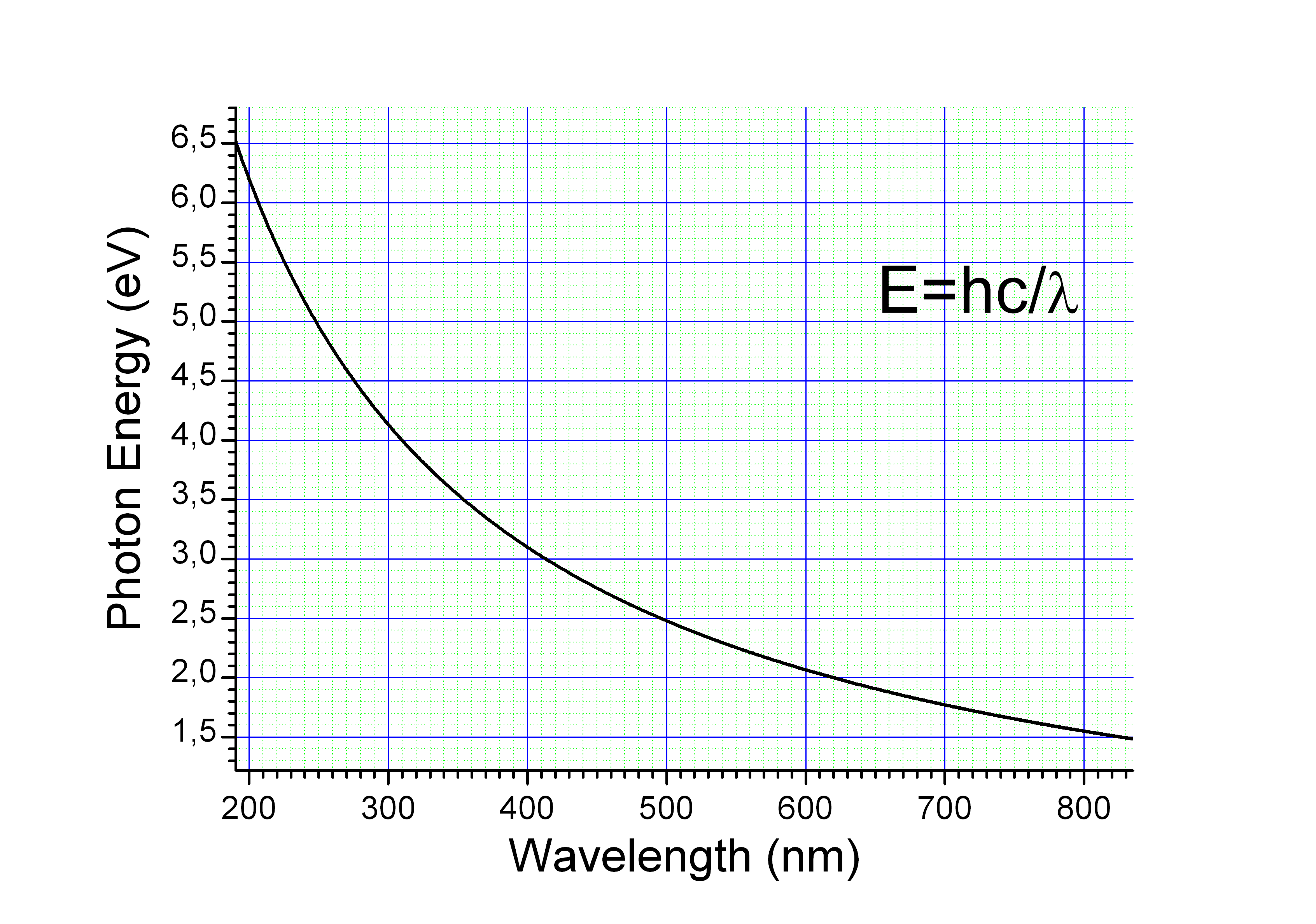
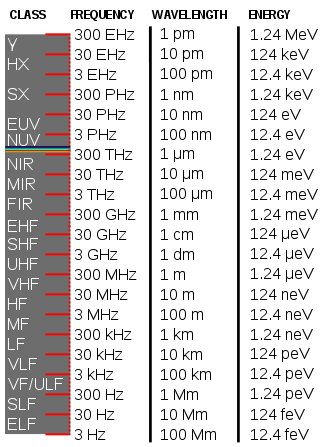
Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download

Calculating particle properties of a wave Ch. 12 A light wave consists of particles (photons): The energy E of the particle is calculated from the frequency. - ppt download