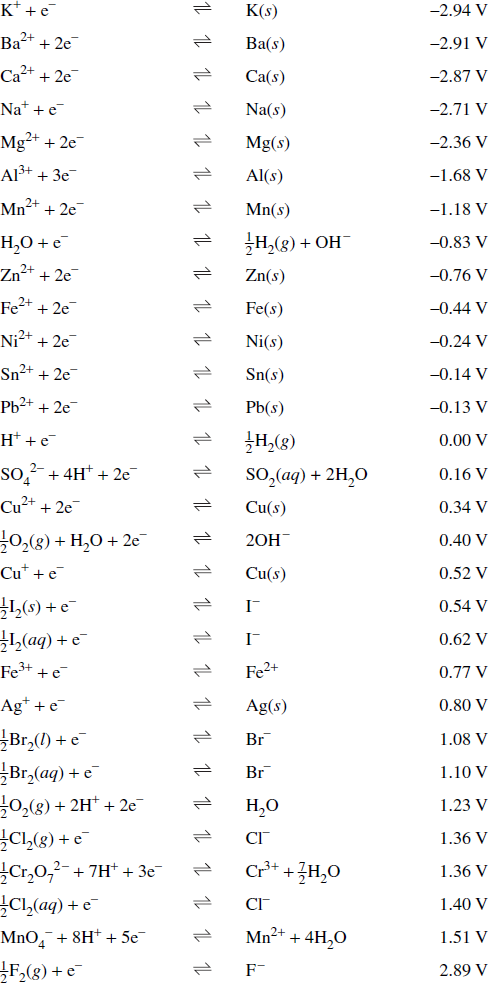![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar

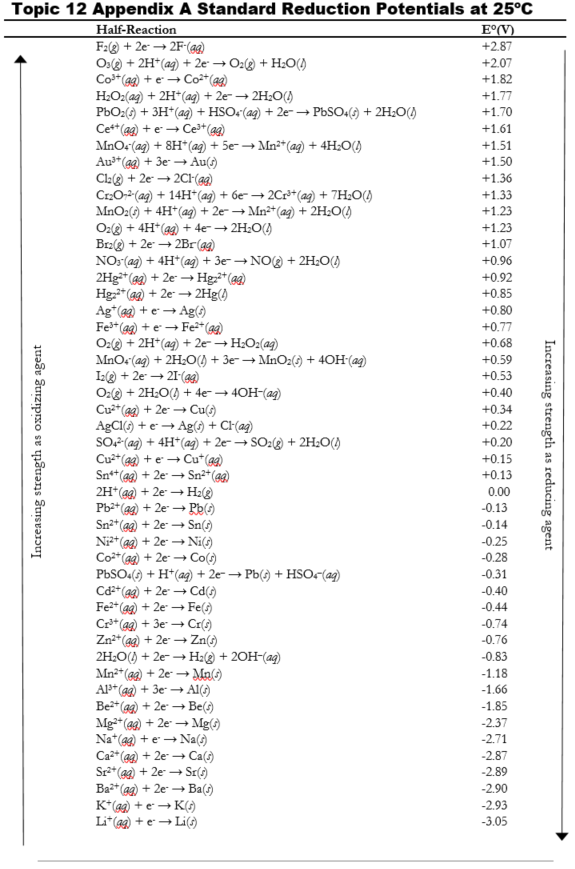
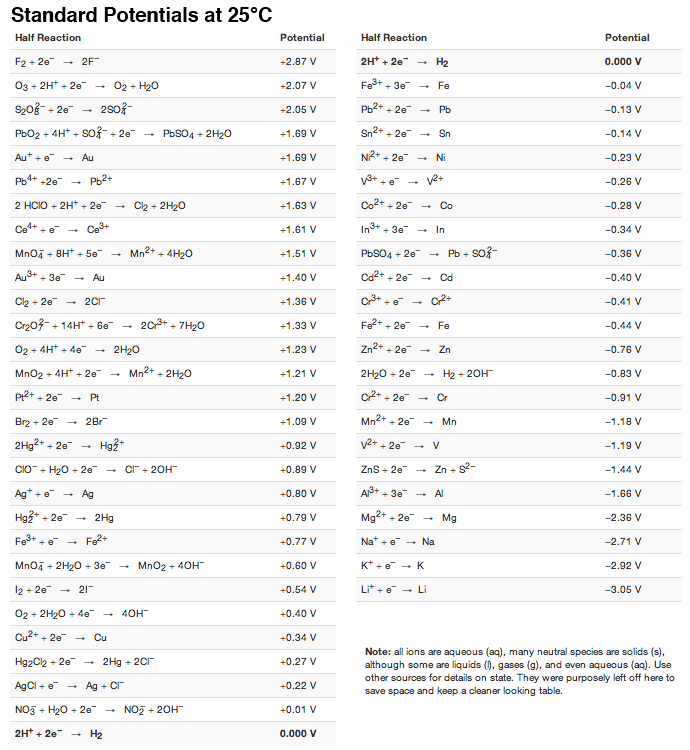
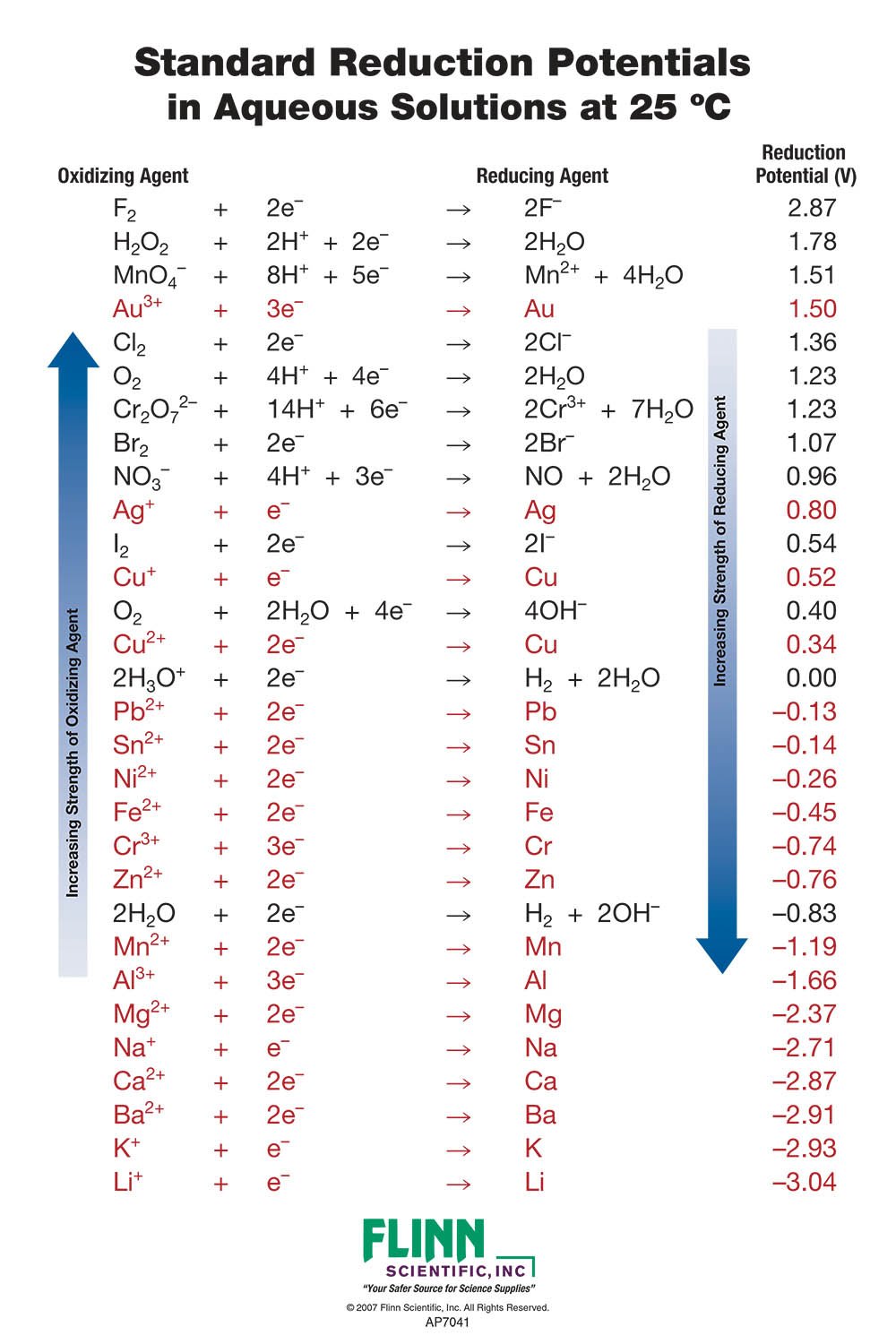
SOLVED: Table A5.5 Standard Reduction Potentials at 25°C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH- + F2 â†' 2F- + 2H2O 2.87 2H2O + 4e- â†' Cu2+ + 2OH- 0.01
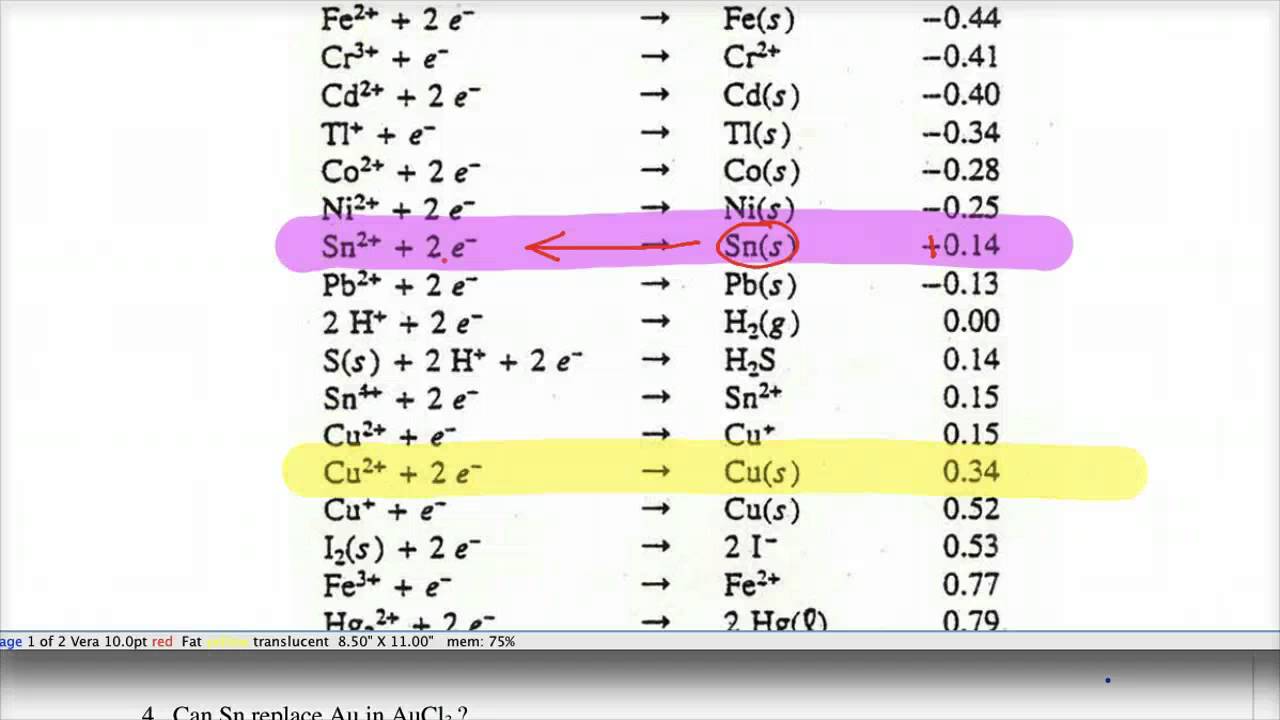
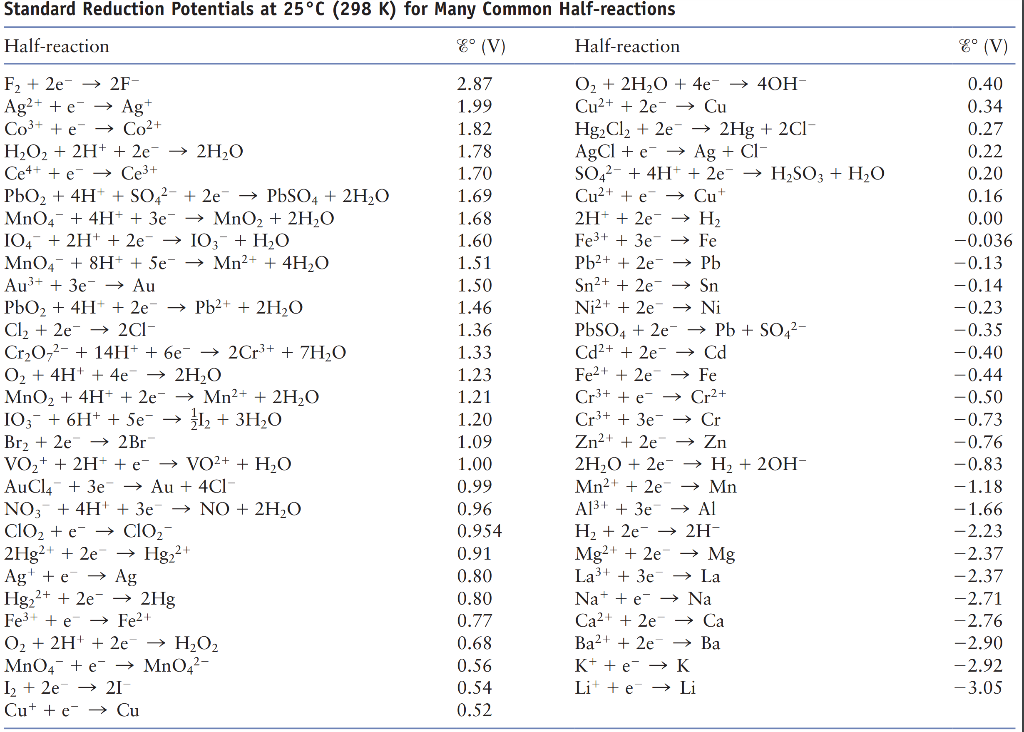
Using the standard electrode potentials given in Table 3.1, predict the reaction between the following is feasible:(i) Fe^{3+}(aq) and I^{-}(aq)(ii) Ag^{+} (aq) and Cu(s)(iii) Fe^{3+} (aq) and Br^{-} (aq)(iv) Ag(s) and Fe^{3+} (

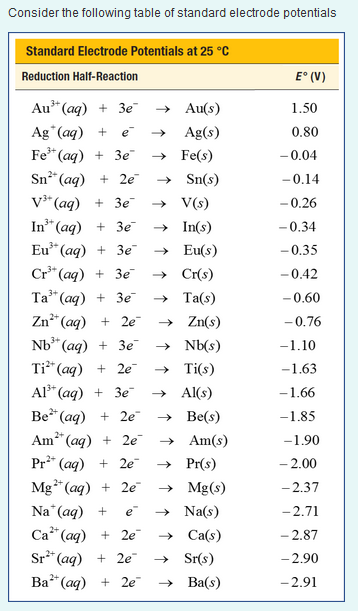
Table of Standard reduction potentials.pdf - Table of Standard reduction potentials Half reaction Li e Li s K e K s Ca2 2e Ca s Na e | Course Hero

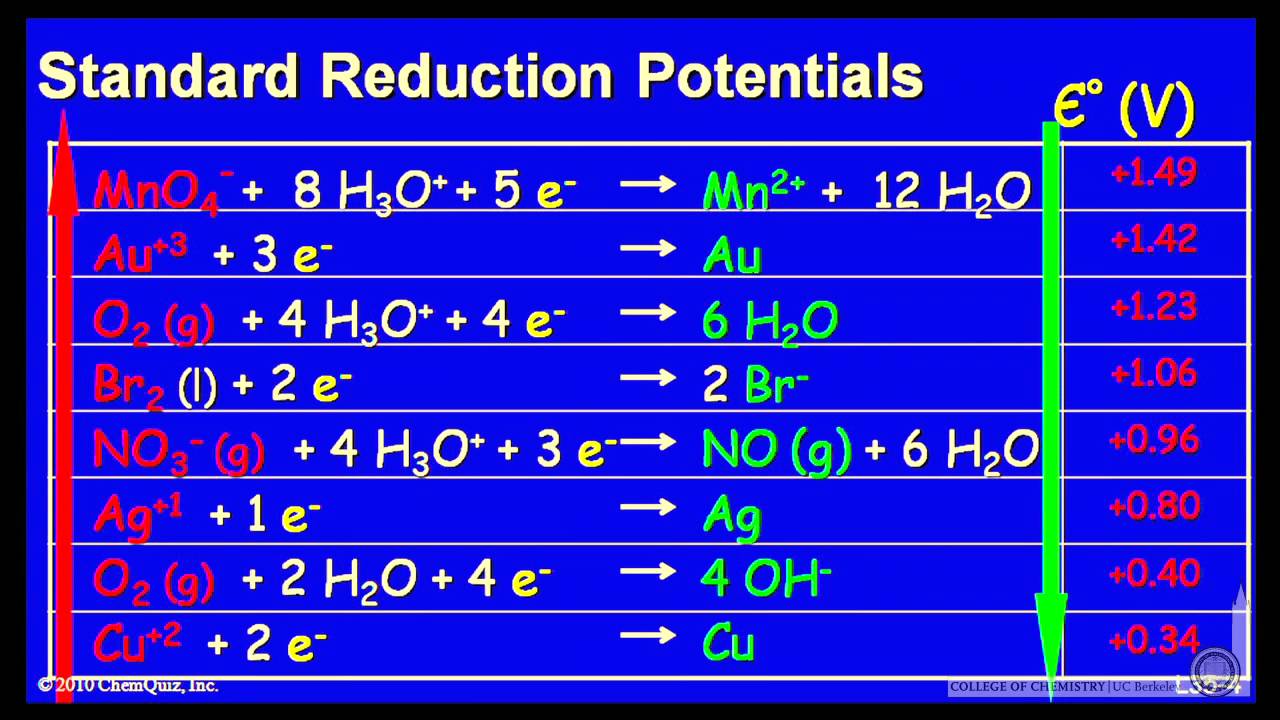
Standard Reduction Potential (E): when given two half reactions and then asked to give E for a reac… | Reduction potential, Chemistry education, Across the universe

Table 1 from Potential-energy and free-energy surfaces of glycyl-phenylalanyl-alanine (GFA) tripeptide: experiment and theory. | Semantic Scholar