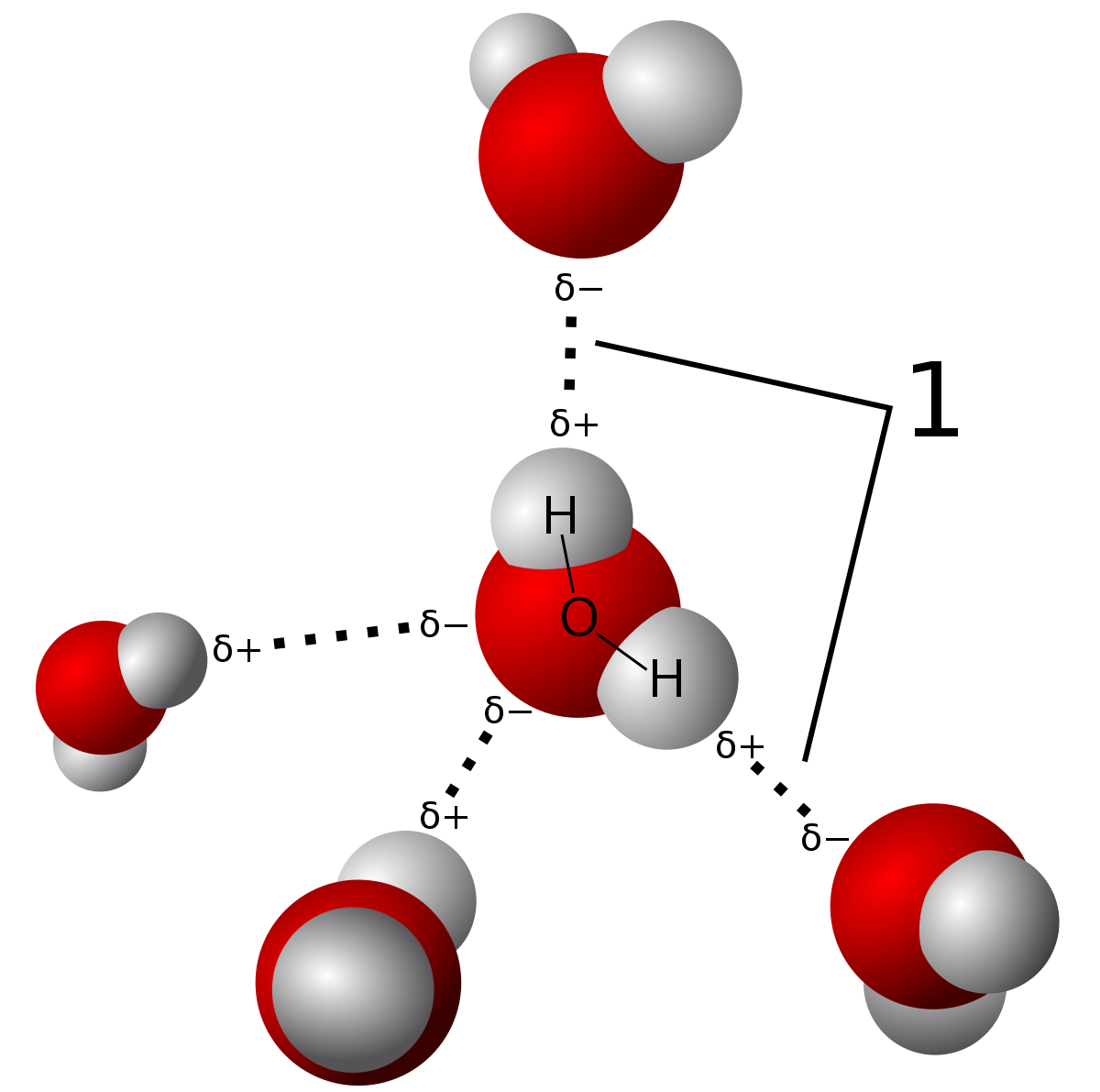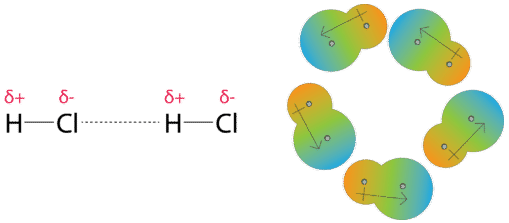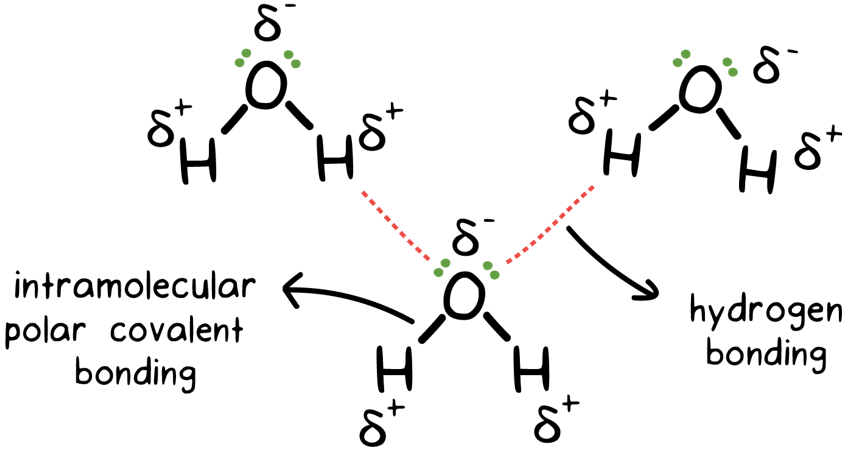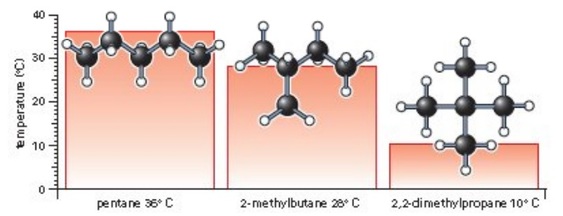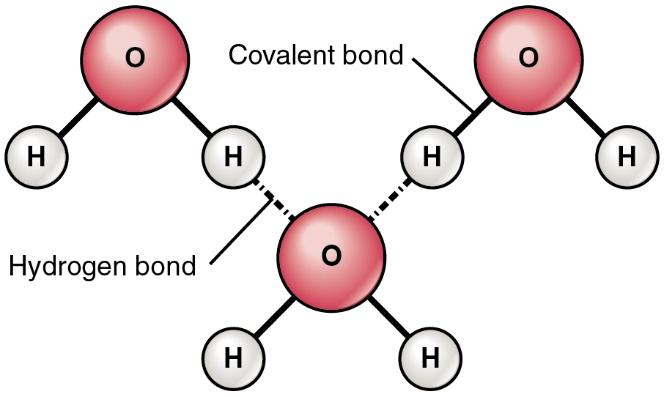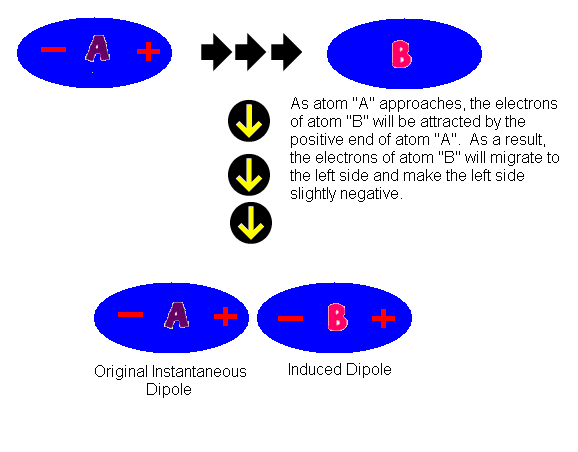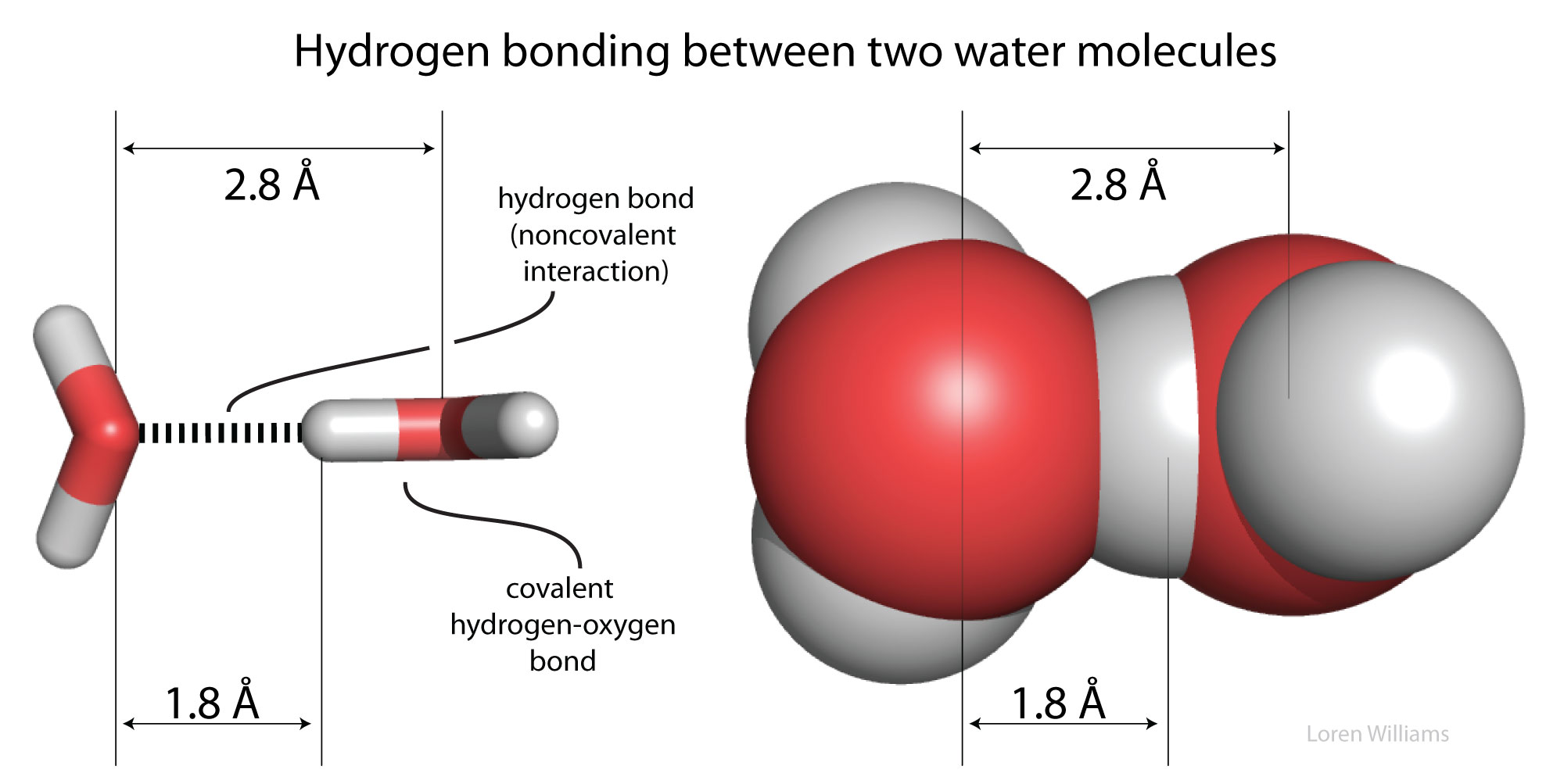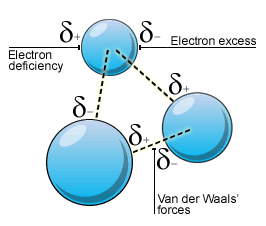
How would you explain the difference between van der Waals interactions and hydrophobic interactions? | Socratic
How would you explain the difference between van der Waals interactions and hydrophobic interactions? | Socratic

Intermolecular Attractions: Attractions between molecules Van der Waals Forces Dipole interactions Dispersion forces Hydrogen Bonds. - ppt download
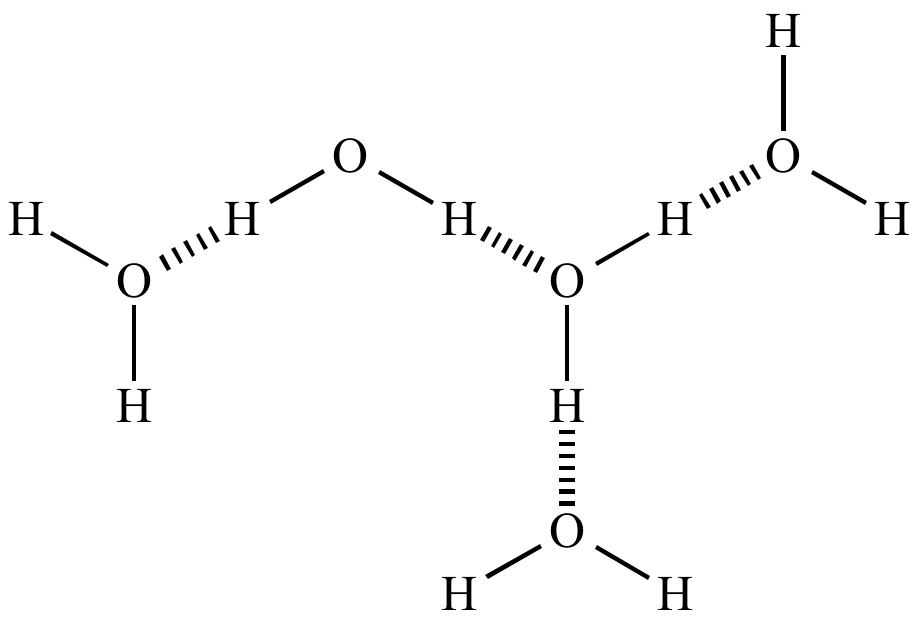
Illustrated Glossary of Organic Chemistry - Noncovalent molecular force; noncovalent molecular interaction; van der Waals forces

While van der Waals forces are normally thought to be repulsive for... | Download Scientific Diagram

Schematic diagram of the interaction forces between the AFM tip and the... | Download Scientific Diagram